See also Sarocladium kiliense, recently changed from Acremonium kiliense.
Showing posts with label conidia. Show all posts
Showing posts with label conidia. Show all posts
Friday, 27 March 2015
Acremonium species
Acremonium species -Hypocreaceae Family (obsolete name Cephalosporium spp.)
Ecology:
Acremonium
species are yet another cosmopolitan fungus (ie. found just about everywhere)
which can be isolated from soils as well as decaying plant material. There are about one hundred recognized species
of Acremonium.
Pathogenicity:
Acremonium
species is associated with ‘white grain mycetoma’[i],
an infection most commonly of the foot. Acremonium has also been implicated in
meningitis, endocarditis, endophthalmitis and corneal ulcers. Onychomycosis or ‘tinea unguium’ (fungal
infections of the nail) may also be caused by localized infection with Acremonium. Disseminated infections are rare and may
result from traumatic injury and may be more likely in immunocompromised hosts.
Macroscopic Morphology:
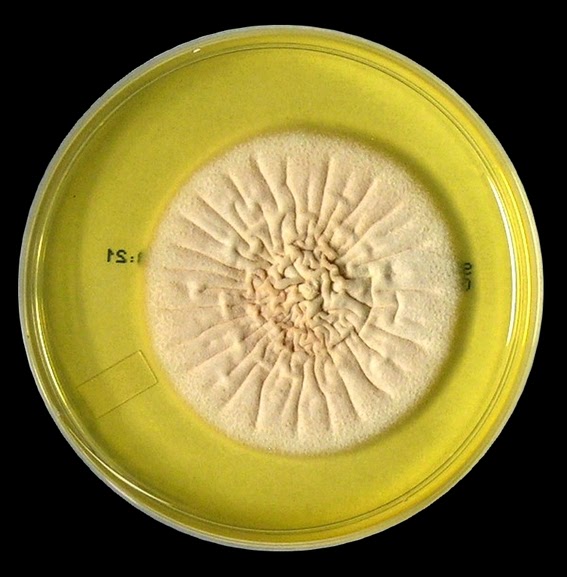
Acremonium exhibits moderately rapid growth, rather flat
colonies that may be slightly raised in the center. The colony has been described as glabrous to
membrane-like initially, becoming powdery, cottony or even felt-like as it
matures. The colony may be white to
cream in appearance or even yellowish to coral or pinkish in colour. The reverse is pale, yellowish to pinkish in
colour.
Acremonium species -Sabouraud Dextrose Agar (SAB) incubated at 30˚C for 18 days. (Nikon)
Acremonium species -Sabouraud Dextrose Agar (SAB) incubated at 30˚C for ~21 days. (Nikon)
Note -On the Challenges of Plate Photography:
This is the same species as above with a few more days of incubation. The reason I posted both these photos is to show the difficulty I have in expressing the true colour (and often texture) of the fungus even when grown on the same media and under identical conditions. The first photo was taken with the plate placed against a white background and the second against a black background.
The other challenge is lighting. I take these photos for my own entertainment and education - for "fun" as the title of the blog states. The acute care lab I work out of is interested only in a quick and accurate identification of fungi in clinical specimens. Documentation by photography is not a concern and we have no professional set up for taking photographs. I have rigged up my own apparatus for use as a camera stand for use within a biological safety cabinet (BSC) (see the post entitled 'Toys'). Photography of macroscopic plates is confined to the BSC as the lab could quickly become contaminated with spores if fungal plates were to be examined outside of the BSC. Many fungi produce vast quantity of spores which can become airborne with only the slightest breeze.
I have little control over the lighting. I often take a large number of photos using both white and black backgrounds. I simply use a sheet of white paper which I have run through a photocopier without a "target". In other words, I leave the cover of the photocopier open and "copy the air" which results in a sheet of paper covered with back toner on one side but white on the back. I can flip this paper over to the black or white side as I wish and as the background paper may be contaminated with spores, I can discard it safely when finished.
I use the fluorescent tube lighting of the BSC itself, I have an incandescent source of lighting I can bring into the cabinet, and I can use the flash unit on the camera itself in order to manipulate lighting. Light coloured fungi often show more detail when on a dark background and dematiacious fungi (darkly pigmented) stand out better on white backgrounds - but not always! I try to avoid cast shadows. I also find that the flash often produces such glare and reflection off of the petrie dish and agar surface that it obscures the true nature of the organism. By taking a large number of photos on various backgrounds and with various combinations of the lighting I have available, I can usually find a photograph that reflects the true characteristics of the fungus I am attempting to document.
Microscopic Morphology:
Acremonium
produces septate hyphae from which erect, unbranched and tapering phialides
extend. Most phialides (but not
necessarily all) have a basal septum which delimits them from the hyphae
proper. Conidia are oblong (2–3 X 4–8
µm) are usually one-celled, however bicellular conidia may occur. The conidia are produced and accumulate as
balls (rarely as chains) at the apices of the phialides but they are fragile
and easily disrupted.
Phialide: A specialized conidiogenous cell (conidiophore) that produces conidia in basipetal succession without increasing in length. (Mycology Online)
Acremonium
species - above is a little piece of agar which adhered to the slide culture microscope cover slip when removed from the agar block (See Post on Slide Cultures). From this little flake of agar you can see phialide extending outwards with little "balls" of conidia attached at their apices. (LPCB, DMD-108, 250X)
Acremonium
species - a collection of hypha run through the center of the photo from which you can see the phialide bearing conidia at their apices. This is like "micro-botany" with the fungi as tiny plants, the 'seeds' (conicia) at the tips of the stems. (LPCB, DMD-108, 400X)
Acremonium
species - As above but at a slightly higher magnification.
(LPCB, DMD-108, 400+10X)
Acremonium
species - the individual conidia can now be seen, gathered at the tapering tips of the phialides. (LPCB, DMD-108, 1000X)
Acremonium
species - not the greatest shot but try to picture the three 'balls' of conidia siting at the top of the phialides which are extending upwards, towards you, the viewer. The phialides are blurred (out of focus) as they are out of the focal plane of the camera, lying beneath the balls of conidia, where they attach to the hyphae. (LPCB, DMD-108, 1000X)
Acremonium
species - The same as above but viewed more from the side. The originating hyphae and tapering phialides are slightly out of the focal plane of the camera. The conidia that were produced at the apex of the phialide remains undisturbed as a ball around the top.
(LPCB, DMD-108, 1000_10X)
Acremonium
species - The previous photos show the phialides and conidia almost in three-dimensions. In the photos that follow, they are lying fairly flat, making it easier to see the features.
(LPCB, Nikon, 400X)
Acremonium
species - in the center of the photograph, there are two phialides, both with a collection of conidia at their apices, where they remain after being produced.
(LPCB, DMD-108, 1000X)
Acremonium
species - another view of the tapering phialides extending from their parent hyphae where elongated, ellipsoidal conidia lay gathered.
(LPCB, DMD-108, 1000X)
Acremonium
species - pretty much the same as in the above photo. The phialide at the very top of the photo appears to have produced only one conidium. Perhaps it is younger than the others.
(LPCB, DMD-108, 1000X)
Acremonium
species - another photo of tapering phialides and the ellipsoidal conidia gathered around the apices. (LPCB, DMD-108, 1000X)
Acremonium
species - insert -one tapering phialide with individual conidia gathered at the apex.
(LPCB, DMD-108, 1000X)
Acremonium
species - Two phialides, side by side producing large quantities of conidia.
(LPCB, DMD-108, 1000+10X)
Acremonium
species - tapering phialide with single ellipsoidal conidia at the apex. A slight collarette can be seen remaining around the apex. Conida already produced almost seem to have fallen down and accumulated around the base of the phialide and hypha.
(LPCB, DMD-108, 1000+10X)
Acremonium
species - as previously -another view of the tapering phialides and the balls of conidia adhering to the apex of the phialides where they were produced.
(LPCB, DMD-108, 1000+10X)
Acremonium
species - As previously.
(LPCB, DMD-108, 1000X)
Acremonium
species - a few barren phialides can be seen and the large mass of dispersed, primarily single celled conidia seen throughout.
(LPCB, DMD-108, 1000X)
Acremonium
species may be confused with Verticillium
and some isolates of Fusarium where
macroconidia are not present. Rate of
growth and colony morphology differs from that of Acremonium species and may provide initial clues for
differentiation.
See also Sarocladium kiliense, recently changed from Acremonium kiliense.
See also Sarocladium kiliense, recently changed from Acremonium kiliense.
[i] Mycetoma, or maduromycosis, is a slow-growing bacterial or fungal infection focused
in one area of the
body, usually the
foot. Approximately
one month or more
after the injury,
a painless nodule forms under the skin surface. The nodules
develop into a tumor which produces sinuses to drain fluid. The
fluid contains tiny
grains, which may be a clue as to the type of organism
is causing the infection.
* * *
Wednesday, 30 October 2013
Exserohilum rostratum
Exserohilum rostratum (Mould)
Ecology:
Exserohilum
species are dematiaceous fungi (ie black mould),
widely distributed in nature. They are
cosmopolitan, commonly found on many plants and grasses and can also be
isolated from soils and water.
Pathogenicity:
Exserohilum species
are unlikely human pathogens. Exserohilum species have been implicated
in phaeohyphomycosis[i].
Most commonly they are isolated from nasal sinuses (sinusitis) and the eye
(keratitis) after a scratch or traumatic injury.
In 2012, Exserohilum rostratum was implicated as
the primary pathogen isolated from injectable methylprednisolone. Numerous patients
receiving steroid therapy primarily for degenerative lumbar-disk and joint
disease in the U.S., developed meningitis after injection. While normally not
invasive, the fungus will cause illness when directly injected into the body. While
sources vary in statistics, injectable methylprednisolone contaminated with Exserohilum rostratum appears to have
been responsible for sickening upwards of 700 people, 30 of which died. Many others continue to have ongoing
neurological problems associated the infection or subsequent therapy.
Macroscopic Morphology:
E.rostratum
exhibits rapid growth and matures within 4 to 5 days.
Surface growth is grey in
colour, quickly darkening with the production of melanin, eventually developing
shades from olive to brown to black.
The texture is woolly or
cottony in appearance.
Exserohilum rotratum SAB 30oC, 72 Hours
Exserohilum rostratum SAB 30oC, 1 Week
Microscopic Morphology:
+Reverse.jpg) Hyphae are septate and
darken with the development of melanin.
Conidiophores are rather long (up to 200 - 230 µm in length, 5 – 8 µm
wide) and are also septate.
Conidiophores exhibit sympodial geniculate growth, where conidia are
produced at bends (geniculate) as the conidiophore extends. This gives the conidiophore a knobby, zigzag
appearance where the conidia attach. The
mature conidia (ave. 14 X 80 µm or greater) are straight to slightly curved and
are fusiform or ellipsoidal in shape with rather smooth walls. Conidia are compartmentalized with between 7
to 11 septa and has a distinctive protruding dark hilum (scar) at the base
where once attached.
Hyphae are septate and
darken with the development of melanin.
Conidiophores are rather long (up to 200 - 230 µm in length, 5 – 8 µm
wide) and are also septate.
Conidiophores exhibit sympodial geniculate growth, where conidia are
produced at bends (geniculate) as the conidiophore extends. This gives the conidiophore a knobby, zigzag
appearance where the conidia attach. The
mature conidia (ave. 14 X 80 µm or greater) are straight to slightly curved and
are fusiform or ellipsoidal in shape with rather smooth walls. Conidia are compartmentalized with between 7
to 11 septa and has a distinctive protruding dark hilum (scar) at the base
where once attached.
More specifically, the
conidia are ‘poroconidia’, a distinction where the conidia are produced through
the extrusion or extension of the inner walls of the conidiogenous cells
through a pore or channel.
Exserohilum rostratum -First look. Free conidia. Tape mount at 250X (LPCB, DMD-108)
Exserohilum rostratum -Conidia attached to septate hyphae (400X, LPCB, Nikon)
Exserohilum rostratum - Large, compartmentalized conida which are somewhat fusiform in appearance and may appear slightly bent or curved. (400X, LPCB, DMD-108)
Exserohilum rostratum - as above. Showing variation in size and shape of conidia. Brown pigmentation due to the production and accumulation of melanin. (400X, LPCB, DMD-108)
Exserohilum rostratum - Conidia attached to conidiophore.
(400X, LPCB, DMD-108)
Exserohilum rostratum - as above, conidia attached to conidophore seen extending through the camera's plane of focus. (400X, LPCB, DMD-108)
Exserohilum rostratum - a closer look at the septate conidiophore and the attachment of the conidia to the conidiophore. Note the bent or zig-zag location on the conidiophore at the point of conidial attachment. This appearance us referred to as geniculate growth. (400X, LPCB, Nikon)
Exserohilum rostratum - Pigmented, fusiform shaped conidia, usually containing between7 to 11 internal septa. Note the prominent projection or hilum (arrow) which remains at the point of the attachment to the conidiophore. (400+10X, LPCB, DMD-108)
Exserohilum roatratum - single conidium with hilum visble on right side. Length reads 76.01
µm. (400+10X, LPCB, DMD-108)
µm. (400+10X, LPCB, DMD-108)
Exserohilum rostratum - another view of the geniculate growth / attachment of the conidia to the conidiophore. (400X, LPCB, DMD-108)
Exserohilum rostratum - Single conidium attached to conidiophore.
(1000X, LPCB, DMD-108)
Exserohilum rostratum - okay, I like photos! Another loose conidium which is slightly bent. This one has seven, possibly eight compartments. The conidium is smooth walled and again, the hilum is clearly visible at one end. (1000X, LPCB, DMD-108)
Exserohilum rostratum - More photos, just for the beauty of this organism.
(1000X, LPCB, DMD-108)
Exserohilum rostratum
Exserohilum rostatum - geniculate (zig-zag) conidiophore after the conidia have dispersed.
(400X, LPCB, DMD-108)
Caution:
Exserohilum species may be
confused with Bipolaris and Drechslera species however Exserohilum has the protuberant hilum.
[i] A
particular presentation of a fungal infection in tissue caused by certain dematiaceous
fungi. This presentation may be an
initial clue as to the particular fungus responsible for the infection. 'Google' the term for a better definition.
* * *
Saturday, 13 April 2013
Trichothecium roseum
Trichothecium roseum (Fungus)
Ecology: Trichothecium species are cosmopolitan
fungi (found just about everywhere) and is a common saprobe (growing on
decaying vegetation). In particular, it
has been isolated from withering fleshy fruits such as peaches, plums and
nectarines. Trichothecium is responsible for ‘pink rot’ of apples.
Macroscopic
Morphology: Trichothecium is a rapidly growing fungus which is fully mature in
3 to 4 days. Colonies are described as
being flat, suede-like to powdery, which start off white but quickly develop a
light pink to peach colour which may reach a darker salmon colour on continued
incubation. The reverse is a rather non-descript
light or pale colour. Pictured below are
colonies grown at 30oC on Sabouraud Dextrose Media (SAB or SDA). Trichothecium
fails to grow at 37oC making it an unlikely candidate for human
pathogenicity.
Trichothecium roseum on SAB agar, 72 hrs, 30oC (Nikon)
It was rather difficult to capture (and correct for) the exact colour hue under harsh labor fluorescent laboratory lighting within the biological laminar flow hood. The colour descriptions range from a light pink to peachy to salmon or even orange on extended incubation.
Trichothecium roseum on SAB after 14 days incubation at 30oC (Nikon)
Microscopic
Morphology: Hyphae produced by Trichothecium are septate and hyaline
(clear, not pigmented). The long, thin and
erect conidiophores are indistinguishable from the vegetative hyphae and may
exhibit septation near their base of attachment. Trichothecium
roseum produces rather thin walled, two-celled conidia (16-20µm X 8-12µm)
which are pyriform or clavate in shape.
Basipetal growth has the newest cell developing below the previous one (The youngest cells are at the base while
the oldest are at the apex.) This
growth produces a sympoidal pattern seen as zigzag or alternating conidia extending
from the conidiophore on opposite sides.
Free conidia have a truncated basal scar usually obliquely offset,
indicating their former point of attachment.
Note: All photos which appear below were taken with the Leica DMD-108 digital microscope.
Trichothecium roseum growing from the surface edge of agar (bottom of photo). Fine hyphae and conidiophores bearing conidia are seen (7 days 250X LPCB)
Trichothecium roseum -conidiophores are seen extending along the lengths of hyphae. Conidia are seen clumped at the apex of the hyphae. Branching of conidiophores is rare if it occurs at all.
(250x. LPCB)
Trichothecium roseum - conidiophores with early production of two-celled conidia are seen extending from a hyphal element just out of focus below. A number of two-celled conidia are seen free of the conidiophore. A truncated basal scare can be seen which may may be somewhat offset from center (arrow), due to the sympodial pattern of growth.
(400X, LPCB)
Trichothecium roseum - again, conidiophores are seen extending from the hyphae (slightly out of focus at top). Cells appear 'clustered' around the apex of the conidiophore as the growth extends. Note 100µm bar at top right of this and previous photo.
(LPCB, 400X)
Trichothecium roseum - conidiophores extending from hyphae with pyrimidal or clavate shaped (pear shaped) conidia extending to the apex. Again, 100µm bar appears on this and various other photos for scale. (400X, LPCB)
Trichothecium roseum - somewhat thick walled, two-celled clavate conidia are seen at the apex of the conidiophore extending upwards into the focal plane of the camera. The conidium at the top of the group appears contorted (twisted) at the bottom where it is attached to the conidiophore. When released, a truncated basal scar will be present at this attachment point and it will be somewhat offset from the centerline of the conidium. (1000X, LPCB)
Trichothecium roseum - once again the clavate shaped conidia are seen attached along the conidiophore. Here you can see the sympodial growth pattern which produces conidia in an alternating or zigzag pattern along the conidiophore. The youngest cells are at the bottom with the most mature at the apex (top) of the cluster. (1000X, LPCB)
Trichothecium roseum - free conidia showing the pyramidal, clavate (club shaped) or perhaps pear shape characteristic of this fungus. The cell wall is rather thin to moderately thickened and the central division is visible in most cells above. Again, the basal scar appears at the previous point of attachment and may be somewhat offset from the center line of the conidium.
(1000X, LPCB)
Trichothecium roseum - clavate two-celled conidia seen attached to conidiophore
(1000+10X, LPCB)
Trichothecium roseum - one more photo just for the heck of it. Septate hyphae can be seen. Sympodial attachment of cells can be seen with the two cells near the center of the photo.
(400X, 14 days, LPCB)
Trichothecium roseum -Computer wallpaper (1024X768)
Pathogenicity: Trichothecium species are generally clinical
laboratory contaminants. No human or
animal infections have been reported.
Differentiation: Trichothecium
roseum may initially be confused with Microsporum
nanum as this fungus produces a similar light pink to buff coloration. M.nanum,
however, exhibits a reddish-brown pigment on reverse in contrast to the pale
reverse of T.roseum. The conidia produced by M.nanum are also two celled however they are sessile (attached directly
to undifferentiated conidiophores) or on short stalks. Finally, Microsporum nanum has the ability to
perforate hair cells and is not inhibited by the cycloheximide in Mycosel agar.
* * *
Subscribe to:
Posts (Atom)



















.jpg)
.jpg)

.jpg)




.jpg)








.jpg)
.jpg)
+14d.jpg)










.jpg)

.jpg)























