Showing posts with label macroconidia. Show all posts
Showing posts with label macroconidia. Show all posts
Sunday, 27 September 2015
Epidermophyton floccosum
Epidermophyton floccosum (mould/dermatophyte)
Note: While I've had photos of Epidermophyton floccosum for some time now, I've never been satisfied with the quality of the microphotographs I've taken. I remain unsatisfied here. The photos for this and every other post contained in this blog were taken by myself on my own time, before or after regular work hours. Unfortunately I find myself so busy at times that my own projects take a distant back seat. Primary cultures sometimes may become contaminated or overgrown. Isolates may revert to a sterile form on repeated subculture, as in this case, before sufficient study. While I've obtained several isolates over the years, E.floccosum always seems to defeat my best efforts to obtain those "text book" quality photos. Time is running out...
Ecology:
Epidermophyton floccosum is a
cosmopolitan (worldwide distribution)
anthropophilic (man is the primary host
& reservoir) dermatophyte [i]
. A once though related species, Epidermophyton stockdaleae has been
determined to be a synonym for Trhycophyton
ajelloi which exhibits no known pathogenicity to humans or animals
Pathogenicity:
E.floccosum
causes tinea pedis (athlete’s foot), tinea cruris (groin infections or
“jock-itch), and tinea corpis (body infections), and to a lesser extent
onychomycosis (nail infections). Skin infections are also known as “ring-worm”
though there is no ‘worm’ involved.
Infection with E.floccosum may
be transmitted in gym facilities where unprotected feet may share a common
floor. E.floccosum rarely infects the
scalp and does not infect hair or hair follicles.
Macroscopic
Morphology:
E.floccosum exhibits moderate growth, becoming mature in about 10 –
14 days. Surface colonies (media
influenced) have been described as mustard yellow or yellowish brown to
olive-grey (khaki) in colour. Colonies
can be powdery, velvety or felty in texture and acquire a folded appearance as
growth progresses. After prolonged
incubation, sterile floccose (hairy)
white mycelia may cover the colony. The
reverse has been described as ochre, mustard-yellow to yellow-brown and even orange
in colour.
E.floccosum -colony heaped up at center on SAB after 2 weeks at 30ᵒC (Nikon)
E.floccosum -colony on SAB (Saboraud Dextrose Agar) after 2 weeks at 30ᵒC (Nikon)
E.floccosum -colony on SAB after 3 weeks at 30ᵒC (Nikon)
E.floccosum -another colony on SAB after 3 weeks at 30ᵒC (Nikon)
E.floccosum -yet another colony on SAB after 5 weeks at 30ᵒC.
Note: white floccose patches beginning to develop. (Nikon)
E.floccosum -colony on SAB, 30ᵒC after repeated subcultures has developed white floccose patches which are areas of sterile hyphae. (Nikon)
Microscopic
Morphology:
E.floccosum has septate hyphae however microconidia are not
produced which differentiates it from the other genera of dermatophytes. Macroconidia develop as lateral or terminal
outgrowths from mature hyphae and initially lacks a basal septum. Rather thin
walled macroconidia (10 -40 µm X 6 – 12 µm) contain 2 to 5 cells can occur singly
or in characteristic clusters. As the
culture ages, macroconidia may transform into chlamydoconidia (chlamydospores) so
they are best observed earlier in growth. The macroconidia are smooth walled,
and clavate (club shaped) with a blunt tip.
This also differentiates it from Microsporum
and Trichophyton. (Again, see endnote 1).
Note:
Stock cultures are best maintained on SAB
media with 3 – 5% sodium chloride. This
may reduce or prevent the isolate from becoming sterile.
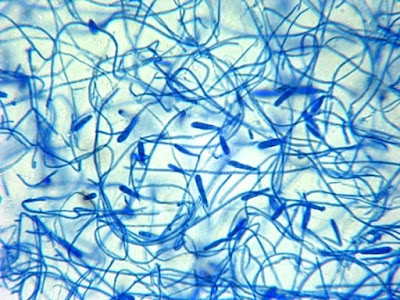
E.floccosum - a first look at low power.
(100X, LPCB, DMD-108)
E.floccosum - Slightly higher magnification reveals the macroconidia more clearly.
(250X, LPCB, DMD-108)
E.floccosum - as above, numerous club shaped macroconidia are clearly seen.
(250X, LPCB, DMD-108)
E.floccosum - club shaped macroconidia with internal septations.
(1000X, LPCB, DMD-108)
E.floccosum - club shaped macroconidia with internal septations.
(1000X, LPCB, DMD-108)
E.floccosum - club shaped macroconidia with internal and basal septations.
(1000+10X, LPCB, DMD-108)
E.floccosum - again, club shaped macroconidia with internal septations. My isolates tended to produce single macroconidia over the grouped macroconidia where several macroconidia crowd each other growing out from the same area of the hypha.
(1000+10X, LPCB, DMD-108)
E.floccosum - a single macroconidium. Note that in this and other microphotographs in of E.floccosum, there are no microconidia.The lack of microconidia is one feature which distinguishes E.floccosum from other dermatophytes.
(1000+10X, LPCB, DMD-108)
E.floccosum - several macroconidia.
(1000X, LPCB, DMD-108)
E.floccosum - a single mature macroconidium.
(1000+10X, LPCB, DMD-108)
E.floccosum - a single mature macroconidium with a curious little kink in it's side.
(1000+10X, LPCB, DMD-108)
E.floccosum - macroconidium measures 35.18 µm in length.
This was obviously an adhesive tape preparation which may trap air bubbles or even reveal uneven adhesive application which may detract from the photograph.
(1000+10X, LPCB, DMD-108)
E.floccosum - club shaped macroconidia. I have this photo recorded as taken at 400X which is confirmed by the micron bar in the upper right. However, the macroconidia seem extremely large for this magnification if compared to previous photos at 1000X. The same goes for the photo which follows. Curious...
(400X, LPCB, DMD-108)
E.floccosum - numerous club shaped macroconidia as above.
(400X, LPCB, DMD-108)
E.floccosum - this photo was taken from a culture that was just over three weeks old. Numerous roundish chlamydospores have developed. Again, compare the micron bar in the upper right to the previous photo which shows identical magnification yet the macroconidia vary greatly is size.
(400X, LPCB, DMD-108)
E.floccosum - macroconidia and chlamydospores present in this adhesive tape preparation
(1000X, LPCB, DMD-108)
E.floccosum - macroconidia on prolonged culture. Some sources say that arthroconidia may also develop, however, I have never observed them in my older E.floccosum cultures.
(500X, LPCB, Nikon)
E.floccosum - finger-like group of macroconidia.
(1000X, LPCB, Nikon)
[i] Dermatophyte – fungi which thrive on
keratin for growth therefore they primarily infect skin, hair and nails
depending on the genera and species. Epidermophyton, Microsporum and Trichophyton
are dermatophytes. Epidermophyton had macroconidia that are clavate (club shaped)
while Microsporum produces fusiform
(spindle shaped) macroconidia and Trichophyton
possesses cylindrical or ‘cigar-shaped’ macroconidia. E.floccosum
does not produce microconida which also serves to differentiate it from the
other dermatophytes.
* * *
Saturday, 19 October 2013
Trichophyton terrestre
Trichphyton terrestre (Fungus/Mould)
Ecology:
Trichphyton terrestre is a cosmopolitan (found
everywhere), geophilic (soil loving) fungus.
It may also be recovered as a saprobe (living on dead
organic matter) on the fur of small animals, presumably picked up from soils.
Pathology:
Trichophyton terrestre fails to grow once 35ºC to
37ºC is reached
As it fails to grow at human body temperature, there have
been no reports of human infection by this fungus, nor has it been implicated
in animal infections.
T.terrestre may occasionally be encountered as a
laboratory contaminant.
Correct identification is important so as to not confuse
it with pathogenic dermatophytes.
Macroscopic
Morphology:
- Trichophyton terrestre exhibits moderate growth at 25ºC, maturing in about 8 days.
- Colonies expanded in diameter rather slowly.
- Colonies were off-white to light in colour.
- Reverse appeared yellowish to ochraceous, or even slightly reddish in colour.
- Texture was felty to powdery
The isolate presented here developed a pale to golden
yellow exudate on prolonged incubation which was not reported in other sources.
Trichophyton terrestre on SAB, 15 days incubation at 30˚C
Trichophyton terrestre on SAB, 25 days incubation at 30˚C
Microscopic
Morphology:
- Microconidia (4 – 7 µm by 1 -5 µm) are tear-drop to slightly club-shaped.
- They are borne directly from the vegetative hyphae or are found on pedicles (stalk).
- Macroconidia (4 – 5 µm by 8 – 50 µm) have smooth, thin walls and usually contain between 2 to 6 cells or divisions internally.
- Macroconidia are cylindrical (parallel sides) or slightly clavate (club) shaped.
There may not be an obvious distinction between what may
be called micro or macro conidia. (ie.
The two are not clearly differentiated.)
Free micro (& macro conidia) exhibit a truncate base
or basal scar at what was their point of attachment.
Trichophyton terrestre - Adhesive tape preparation, 250X, LPCB (Nikon)
Trichophyton terrestre - Branching with development of Macro & Micro Conidia.
LPCB 400X (DMD-108)
Trichophyton terrestre -as above
LPCB 400X (DMD-108)
Trichophyton terrestre - free macro & micro conidia
400+10X, LPCB (DMD-108)
Trichophyton terrestre - Conidia stain darker with the LPCB than the hyphae usually do
1000X, LPCB (DMD-108)
Trichophyton terrestre - Here again, the conidia at the tips of the hyphae (conidiophore) can be seen as staining a darker blue than the hyphae themselves. Measurements shown (inset) are for the conidia and hyphe. I regret that I didn't just measure the length of the conidia alone.
1000X, LPCB (DMD-108)
Trichophyton terrestre -extensive branching at near right angles.
1000X, LPCB (DMD-108)
Trichophyton terrestre -more of the same. Darker blue conidia are seen at the end of the hyphae, branching at near right angles.
1000X, LPCB (DMD-108)
Trichophyton terrestre - divisions can be seen in some of the developing conidia (macroconidium)
1000X, LPCB (DMD-108)
Trichophyton terrestre -conidia staining a darker blue with the LPCB stain. Divisions can be seen in the conidia. The one on the lower left of the photo clearly has two.
1000X, LPCB (DMD-108)
Trichophyton terrestre -free macroconidium (4 septations or 5 compartments)
1000X, LPCB Nikon (appears larger due to cropping of photo)
Physiological
Characteristics:
- Hair perforation test is POSITIVE
- Urease is POSITIVE
- BCPCG Media reaction is POSITIVE
- No growth at 35ºC to 37ºC
Trichophyton Agars:
Good growth on all Trichophyton agars. No special growth requirements are required
for growth.
Caution: on early growth, the fungus may somewhat
resemble a Chrysosporium
species. Chrysosporium’s conidia generally do not exceed two cells in
length.
* * *
Sunday, 14 April 2013
Trichophyton mentagrophytes Complex
Trichophyton mentagrophytes Complex (Fungus,
Dermatophyte)
Ecology: T.mentagrophytes
is recognized as having two variants.
Anthropophilic isolates prefer man to animals while the zoophilic
isolates primarily infect animals. Small
rodents appear to be the primary reservoir for the animal variety. Trichophyton
mentagrophytes is a cosmopolitan fungus (found everywhere).
Macroscopic
Morphology: T.mentagrophytes exhibits moderately rapid growth and matures
within 6 – 10 days. Sources have
previously described anthropophilic isolates having a downy, powdery or even
fluffy texture while zoophilic isolated were more granular in appearance. Colonies may vary in colour from white to cream
or yellowish. The reverse also can vary
from yellow to reddish brown to brown or ochre, depending on isolate and
medium.
Trichophyton mentagrophytes -5 days growth on SAB at 30oC
Trichophyton mentagrophytes - 14 days growth on SAB at 30oC
Microscopic
Morphology: Trichophyton mentagrophytes produces septate hyphae from which
branched conidiophores extend. Sessile (not on stalk) microconidia are produced
in rather dense, grape-like clusters on the conidiophores. The microconidia (~2µm to 4µm) are spherical
to pyriform in shape. Macroconidia (20-50µm
to 6-8µm) are cigar to club shaped and may show exhibit some distortion. Macroconidia have a smooth exterior and are
thin walled, usually have between 3 to 8 cells dividing the interior. Macroconidia may be found more readily in
younger cultures. Production of both
micro & macro conidia may vary with the isolate. Coiled or spiral hyphae may be present and in
some strains, structures described as nodular bodies or chlamydospores may be
present.
Note: All photos which follow were taken with the DMD-108 digital microscope.
T.mentagrophytes showing sessile microconidia along septate hyphae.
Note 100µm bar in upper right of this and several other photos.
(400x, LPCB)
Trichophyton mentagrophytes - branched conidiophores bearing spherical conidia in clusters seen extending from septate hyphae. (400x, LPCB)
T.mentagrophytes - another view as above. A macroconidium can be seen near the lower center of the photo (400x, LPCB)
T.metagrophytes - a closer look at the branched condiophores bearing clusters of spherical microconidia. Septations are visible in the hyphae and conidiophores.
(1000x, LPCB)
T.mentagrophytes -another look as in the previous photo.
(1000x, LPCB)
T.mentagrophytes - A 7-celled macroconidium. Macroconidia are typically described as being cigar shaped or club shaped.
(400x, LPCB)
T.mentagrophytes - another 7-celled macroconidium with dimensions (inset)
(400+10x, LPCB)
T.mentagrophytes -an macroconidium which shows slight distortion (sides are not straight). Numerous spherical microconidia in lower right.
(1000x, LPCB)
T.mentagrophytes - a solitary cigar shaped macroconidium showing seven internal cells. Walls are rather thin and the exterior is smooth.
(1000+10x, LPCB)
T.mentagrophytes - just by chance all this, and the previous macroconida all contain seven cells. I found that young cultures (~3 days) produced the most macroconidia which seemed to diminish with additional incubation. That said, the macroconidium pictured here is from a 6 day old slide culture. It should always be kept in mind that structures may appear, disappear, develop or change with length of incubation. It may be advisable to make several side cultures and harvest them at different time periods to observe development of structures.
(1000+10x, LPCB)
T.mentagrophytes - a couple of macroconidia are seen in this photo as well as clusters of microconidia. A spiral hyphal element is seen in the upper center of the photo.
(400+10x, LPCB)
T.mentagrophytes - a spiral hyphae is seen seen in the center left of this photo as it overlaps a macroconidium. Microconidia throughout the photo.
(1000x, LPCB)
T.mentagrophytes - more examples of spiral hyphae typical to T.mentagrophytes seen in this photo.
(400+10x, LPCB, 8 days incubation)
T.mentagrophytes - one last photo showing what is described as a nodular body or chlamydospore. Clusters of microconidia seen in center-left.
(1000+10x, LPCB, 10 days incubation)
Physiological
Tests: a number of classical tests can
be employed to speciate Trichophyton
species.
·
Urease test: Positive
·
BCP-Milk Solids Glucose: Alkaline reaction
·
Hair perforation test: Positive
·
Growth at 37oC: Excellent
·
Growth factor requirement*: None
*a variety of Trichophyton
tubed agars are commercially available containing specific growth supplements
(eg.inositol, thiamine, nicotinic acid, histidine). The pattern or degree of growth in each can
assist the speciation of Trichophyton.
Pathogenicity: The anthropophilic strains are usually
associated with chronic infections of glabrous skin, scalp, beard, nails and
feet.
It is currently recommended* that Trichophyton mentagrophytes be reported as ‘Trichophyton mentagrophytes complex’ which also includes the
former Trichophyton krajdenii.
*Best Practice by QMP-LS, external quality assessment
agency.
* * *
Subscribe to:
Posts (Atom)





































.jpg)
.jpg)
.jpg)
.jpg)















.jpg)























